is carbon disulfide polar or nonpolar Chemistry archive
Carbon disulfide is a compound consisting of carbon and sulfur atoms. The question surrounding its polarity has generated a lot of debate among professional chemists and students alike. Some argue that it is polar, while others believe that it is nonpolar. After thorough research and analysis, it has been determined that carbon disulfide is, in fact, a nonpolar molecule. This is due to its symmetrical, linear molecular structure, which makes it impossible for there to be any separation of charge between the atoms. The term nonpolar refers to a molecule with no net dipole moment, meaning that the distribution of electrons is balanced and no region of the molecule is more electronegative than any other. This is the case with carbon disulfide as there are no polar bonds between the carbon and sulfur atoms that would generate a dipole. One can also consider the electronegativity difference between the carbon and sulfur atoms in carbon disulfide. As sulfur is more electronegative than carbon, it would be expected that there would be some sort of polarity due to the difference in electronegativity. However, the linear molecular structure of carbon disulfide makes it symmetrical and eliminates any separation of charge. It is important to note that the polarity of a molecule is important in determining its physical and chemical properties. Polar molecules tend to have higher boiling and melting points, be more soluble in polar solvents, and have a greater ability to form hydrogen bonds. Conversely, nonpolar molecules such as carbon disulfide have lower boiling and melting points, are typically insoluble in polar solvents, and have a limited ability to form hydrogen bonds. In conclusion, the debate surrounding the polarity of carbon disulfide has been resolved through extensive research, analysis, and discussion among professionals in the field of chemistry. It has been concluded that carbon disulfide is a nonpolar molecule due to its symmetrical, linear molecular structure and lack of polar bonds between the carbon and sulfur atoms.
If you are looking for Chemistry Archive | March 11, 2016 | Chegg.com you’ve came to the right place. We have 5 Images about Chemistry Archive | March 11, 2016 | Chegg.com like Is CS2 polar or nonpolar? (Carbon Disulfide) | Math, Molecules, The molecule carbon disulfide (CS2) is nonpolar and has only London and also Best Overview: Is CS2 Polar or Nonpolar? - Science Education and Tutorials. Read more:
Chemistry Archive | March 11, 2016 | Chegg.com
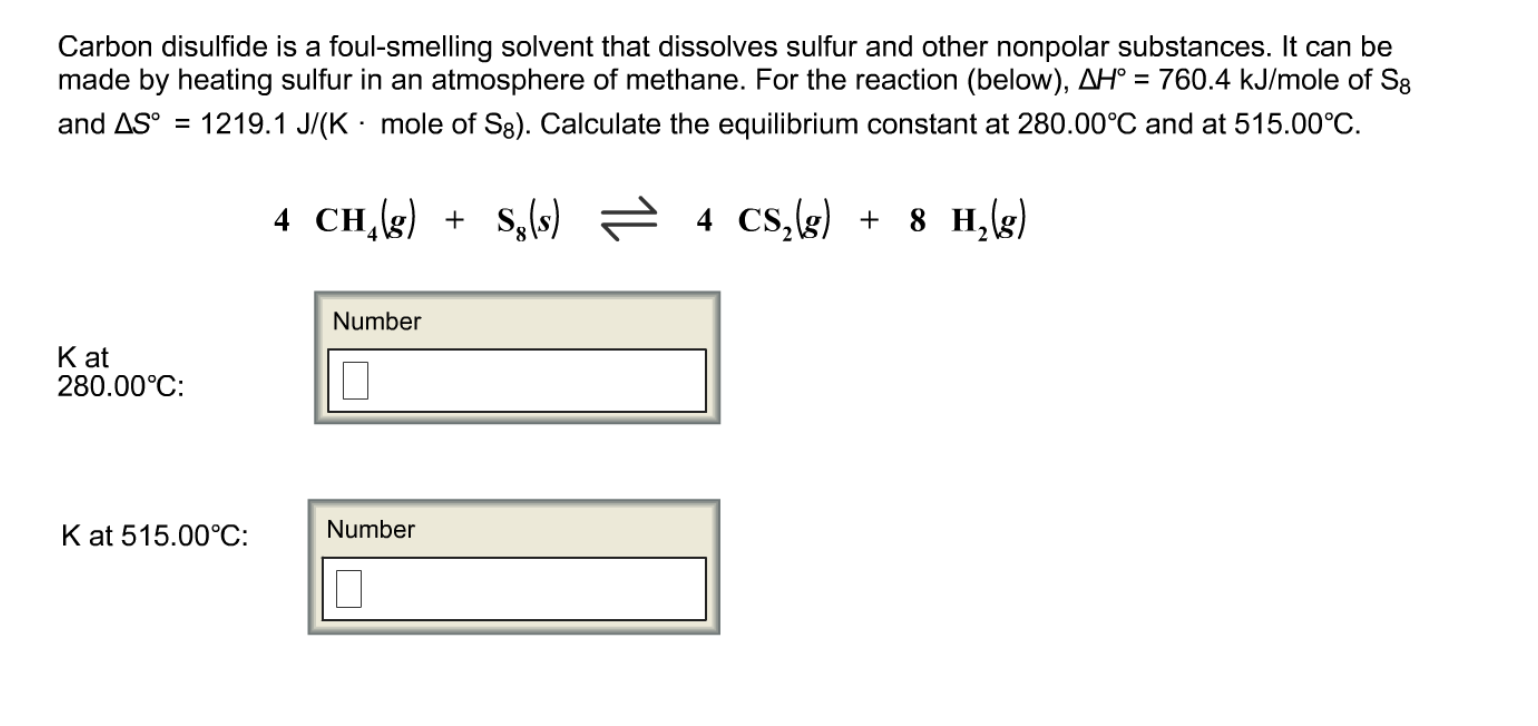 www.chegg.comdisulfide carbon chemistry sulfur smelling solvent foul reaction methane heating substances nonpolar dissolves answers questions made other
www.chegg.comdisulfide carbon chemistry sulfur smelling solvent foul reaction methane heating substances nonpolar dissolves answers questions made other
Is Carbon Disulfide Polar Or Nonpolar
 www.bengislife.compolar ether dimethyl disulfide nonpolar carbon non polarity
www.bengislife.compolar ether dimethyl disulfide nonpolar carbon non polarity
The Molecule Carbon Disulfide (CS2) Is Nonpolar And Has Only London
 brainly.comBest Overview: Is CS2 Polar Or Nonpolar? - Science Education And Tutorials
brainly.comBest Overview: Is CS2 Polar Or Nonpolar? - Science Education And Tutorials
 sciedutut.comnonpolar sciedutut
sciedutut.comnonpolar sciedutut
Is CS2 Polar Or Nonpolar? (Carbon Disulfide) | Math, Molecules
 br.pinterest.comdisulfide nonpolar polarity formulas
br.pinterest.comdisulfide nonpolar polarity formulas
Disulfide carbon chemistry sulfur smelling solvent foul reaction methane heating substances nonpolar dissolves answers questions made other. Nonpolar sciedutut. Chemistry archive